ChemVLab+: Helping students think like chemists
By Sierra McCormick, Jacklyn Powers, Jodi Davenport and David Yaron
Did you attend high school when chemistry instruction focused on isolated facts and procedures without any real-world context? This traditional form of instruction left students without the tools to think conceptually like a chemist. However, in today’s science classrooms, with the integration of the Next Generation Science Standards (NGSS), teachers are encouraged to guide instruction through real-world phenomena and promote critical thinking through inquiry-based learning that helps students learn the reasoning and practical skills of scientists, rather than just rote memorization of scientific facts (NGSS Lead States, 2013).
In alignment with NGSS and funded by the U.S. Department of Education, ChemVLab+ offers a series of freely available, online activities for high school students focused on what chemists actually do: analyze and explain observable events by connecting them to invisible processes at the molecular level. Using authentic scenarios presented through animated videos, the activities allow students to perform experiments in a virtual chemistry lab and engage in problem solving with immediate feedback.
These interactive, self-contained activities for either remote or in-person chemistry instruction are a free resource for teachers. Eight ChemVLab+ activities are available on Carnegie Mellon University’s Open Learning Initiative (http://k12.oli.cmu.edu/). Each activity takes 45 to 90 minutes to complete, and students can pause and resume their investigations at any point. The activities can be administered in any order, but it is recommended that students start with the sports drink activity to best scaffold virtual lab use. For more information on the individual activities and how to access them, visit chemvlab.org.
Activity Snapshot: Optimizing Solar Plant Design
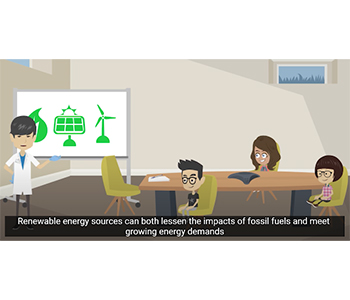
The learning objectives of the Solar Plant Design activity involve understanding heat transfer, heat capacity, and experimental design. The activity begins with animated videos, describing the context: three students visiting a solar power plant to learn about climate change and the increased need for renewable energy (see Figure 1). A scientist details the design of the plant, in which thermal energy from the sun is transferred to a liquid and converted to electrical power. Students then set out to determine the optimal liquid for heat transfer. During classroom use, one student remarked, “It was interesting to have that connection to the solar panels and the whole set up. I liked that part.
presented through animated videos
The learning objectives of the Solar Plant Design activity involve understanding heat transfer, heat capacity, and experimental design. The activity begins with animated videos, describing the context: three students visiting a solar power plant to learn about climate change and the increased need for renewable energy (see Figure 1). A scientist details the design of the plant, in which thermal energy from the sun is transferred to a liquid and converted to electrical power. Students then set out to determine the optimal liquid for heat transfer. During classroom use, one student remarked, “It was interesting to have that connection to the solar panels and the whole set up. I liked that part.”
Following this initial set up, students connect what is observable (temperature) with submicroscopic events (kinetic energy of particles) and determine the direction of heat transfer. Once scaffolded, they are introduced to an initial experiment: determine if three different liquids store heat in the same way. They first design their investigation by selecting the independent variable (type of liquid), dependent variable (final temperature), and constants (initial temperature, heat added, mass). On the following page, they carry out the experiment in the virtual lab, obtaining solutions from a stockroom, placing them over a Bunsen burner for a select number of seconds, and recording their final temperature. Figure 2 shows one student’s virtual lab workspace.
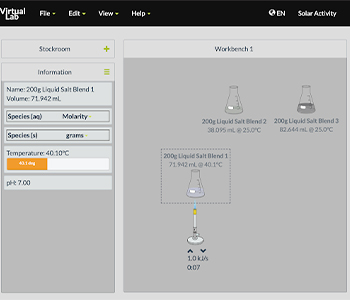
three different liquid salt blends react to added heat from a Bunsen burner.
Students analyze the results, determine how adding heat affects the temperature of solutions, and connect temperature change to the kinetic energy of particles. In the pages that follow, students are introduced to specific heat capacity and mass in the context of this scenario, which prompts them to design and carry out investigations for evaluating those variables. Finally, students utilize the data derived from the investigations to calculate the amount of heat stored by each substance and identify the most optimal liquid for the solar plant.
Students analyze the results, determine how adding heat affects the temperature of solutions, and connect temperature change to the kinetic energy of particles. In the pages that follow, students are introduced to specific heat capacity and mass in the context of this scenario, which prompts them to design and carry out investigations for evaluating those variables. Finally, students utilize the data derived from the investigations to calculate the amount of heat stored by each substance and identify the most optimal liquid for the solar plant.
Activity Design Principles
The ChemVLab+ activities were designed with four principles in mind: use authentic contexts, help students connect multiple representations, provide formative assessment with feedback, and integrate science practices.
Authentic Contexts
Research demonstrates that context-based instruction promotes learning and higher-order thinking skills, as students are more likely to connect their lived experiences with classroom practice (Davenport, Rafferty, Yaron, 2018; Prins, Bulte & Pilot, 2016). By using familiar and motivating scenarios that integrate other disciplines, including environmental science and health medicine, students gain experience using their knowledge to address questions relevant to their lives, such as determining the safety of drinking water, designing medical drugs, or addressing climate change. Animated videos frame content and guide students in an accessible, conversational style.
Multiple Representations
Designing chemical experiments at the high school level requires students to connect what can be controlled and measured at the macroscopic scale to the underlying molecular processes (Clark & Mayer, 2016; Davenport, Rafferty, & Yaron, 2018). The ChemVLab+ activities include features that help students visualize invisible processes and connect them to observable events. As shown in Figure 3, the virtual lab enables students to combine solutions, control the quantities, and then observe reactions through a particulate viewer. As chemists rely on numerous symbols or notations to represent submicroscopic particles, the activities incorporate multiple symbolic representations that illustrate particulate structure, movement, and interactions. Figure 4 shows one exercise in which students match multiple molecular notations with the appropriate chemicals in their study of color change reactions.
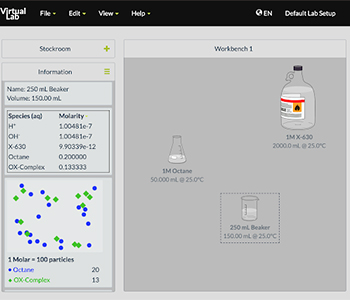
chemical reactions at the submicroscopic and macroscopic scale.
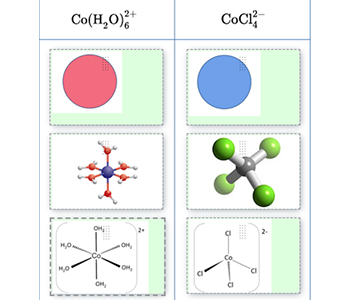
representations that depict particles in a color
change reaction
Formative Assessment with Feedback
Research on assessment demonstrates that interactive, just-in-time assessments can be far more effective in promoting science learning compared to static and distanced formats (Davenport & Quellmalz, 2017). Accordingly, the ChemVLab+ activities provide individualized instruction tailored to each student’s interactions within the activity. Students can request hints and will receive feedback with detailed explanations that allow students to assess their own progress and learning. Instructors can utilize an additional feature called the “Learning Dashboard” that enables them to monitor whole-class or individual progress, check for understanding, and identify learning gaps. As one teacher reported, “I used the reports to check on understanding and participation. They are good for a quick snapshot of progress.”
Science Practice Integration
The Next Generation Science Standards emphasize learning core concepts by engaging in science practices such as asking questions, using models, and carrying out investigations. These practices require students to have access to either physical or virtual environments where they can manipulate systems, gather data, and analyze results. The ChemVLab+ activities provide this opportunity when setting up a physical lab is not possible or practical. Unlike other virtual environments, these labs are embedded in self-contained instructional modules with guiding questions that structure investigations. The open-ended nature of the activities enables students to design and analyze results from their own experiments. Table 1 details student interactions as they relate to NGSS science practices.
Science Practice Integration
The Next Generation Science Standards emphasize learning core concepts by engaging in science practices such as asking questions, using models, and carrying out investigations. These practices require students to have access to either physical or virtual environments where they can manipulate systems, gather data, and analyze results. The ChemVLab+ activities provide this opportunity when setting up a physical lab is not possible or practical. Unlike other virtual environments, these labs are embedded in self-contained instructional modules with guiding questions that structure investigations. The open-ended nature of the activities enables students to design and analyze results from their own experiments. Table 1 details student interactions as they relate to NGSS science practices.
| Science Practices | Student Interactions |
|---|---|
| Developing and Using Models | Students link particulate views with macroscopic events and connect multiple symbolic representations, such as illustrations of molecules with chemical equations. |
| Planning and Carrying Out Investigations | Students design experiments by selecting independent and dependent variables as well as constants. They use the virtual lab activities to explore chemical reactions and make solutions with specified concentrations or molarities. |
| Analyzing and Interpreting Data | Students interpret the results of the virtual lab, analyzing the effects of variables, types of reactions, or system changes, and make conclusions based on their findings. |
| Using Math and Computational Thinking | Students perform calculations and unit conversions, balance chemical equations, and reason about proportional relationships, related to concentration, moles, and mass. |
| Constructing Explanations | Students explain their process, why changes occur, and evaluate other findings or explanations. |
| Obtaining, Evaluating, and Communicating Information | Students evaluate their investigations in the context of the real-world scenario and make recommendations, such as an optimal salt to use for an instant cold pack. |
ChemVLab+ is tested by students and teachers
A study with 1400 high school students found that students using the activities demonstrated increased learning as evidenced by improved problem solving and inquiry over the course of the activities and by statistically significant improvements from pre- to posttest (Davenport & Quellmalz, 2017; Davenport et al., 2018). Researchers developed a second iteration, in collaboration with practicing chemists and teachers, to deepen and expand content that addresses the three-dimensional learning of NGSS. The second iteration, described in this article, was tested for usability and feasibility in 14 classrooms with 1100 students. During the 2020-21 school year, a randomized control trial design pilot study was conducted with 825 students. Analyses are currently underway to explore student learning outcomes.
We are excited that the ChemVLab+ activities can offer a unique way for students to actively engage in science practices using a virtual chemistry lab during remote or in-person learning.
Grant Information
The research reported here was supported by the Institute of Education Sciences, U.S. Department of Education, through Grants R305A100069, R305A170049 to WestEd. The opinions expressed are those of the authors and do not represent views of the Institute or the U.S. Department of Education.
Authors’ Bios
Sierra McCormick is a WestEd Learning and Technology Research Assistant and can be reached at smccorm@wested.org
Jacklyn Powers is a WestEd Learning and Technology Research Associate and can be reached at jpowers@wested.org
Jodi Davenport is the WestEd Director of Learning and Technology and can be reached at jdavenp@wested.org
David Yaron is a Professor of Chemistry at Carnegie Mellon University and can be reached at yaron@cmu.edu
References
Clark, R. C. and R.E. Mayer. 2016. E-Learning and the Science of Instruction: Proven Guidelines for Consumers and Designers of Multimedia Learning. Hoboken, NJ: John Wiley & Sons.
Davenport, J.L., and E.S. Quellmalz. 2017. Assessing Science Inquiry and Reasoning Using Dynamic Visualizations and Interactive Simulations. In Learning from dynamic visualizations: Innovations in research and application, eds. R. Lowe and R. Ploetzner, pp. 203–232. Springer.
Davenport, J.L., A.N. Rafferty, and D.J. Yaron. 2018. Whether and How Authentic Contexts Using Virtual Chemistry Lab Support Learning. Journal of Chemical Education 95 (8), 1250–1259
NGSS Lead States. 2013. Next Generation Science Standards: For states, by states. Washington, DC: The National Academies Press.
Prins, G. T., A.M.W Bulte, A. Pilot. 2016. An Activity-Based Instructional Framework for Transforming Authentic Modeling Practices into Meaningful Contexts for Learning in Science Education. Science Education 100 (6), 1092−1123.






